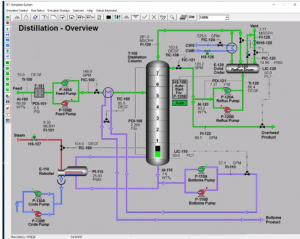
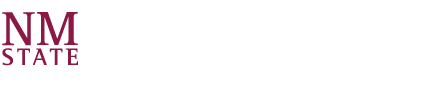The CHME Laboratory provides hands on instruction in the areas of instrumentation, transport phenomena, control systems, data collection and analysis, process safety, and unit operations.
 |
 |
Vapor/Liquid Equilibrium (CHME 323L)
Armfield TH3 Saturation Pressure rig
Description: In this experiment, students are introduced to the fundamental principles of thermodynamics. Experiments begin with basic concepts such as temperature and pressure measurement followed by introduction to the relationships between these concepts as well further study into the first and second laws of thermodynamics, the principles of reversibility, entropy, enthalpy, and more.
 |
 |
Linear and Radial Heat Conduction (CHME 323L)
Armfield Models HT10XC, HT11C, and HT12C
Description: This experiment consists of two separate but related parts. In one of them, students measure the thermal conductivity in the radial direction of a disk of stainless steel. Heat is applied to the center of the disk while the edge is cooled by flowing water. Students set the flowrate of cooling water and the power level of the heater to obtain a steady-state condition, and they measure temperatures at several positions along the radial direction from the center of the disk to the edge using thermocouples embedded in the metal and read with a control unit.
In the other part of the experiment, students measure the thermal conductivity in the axial direction of three different metals (aluminum, brass, stainless steel) of cylindrical shape. Similarly to the radial heat conduction set-up, thermocouples arranged along the lengths of the metals provide temperature data to a control unit.
The control unit is connected to a computer where all data is collected in spreadsheet form and is used by the students to calculate the various thermal conductivities, who then compare them to literature values.
 |
 |
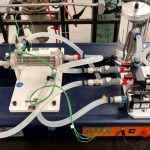
Thermocouple/Viscosity Study (CHME 323L)
Cannon Model 2020 Viscometer
Description: This experiment consists of two parts. In the first, students construct a Type J or Type K thermocouple using the appropriate wire materials and a small welder. They then calibrate this thermocouple by immersing it in water and measuring its voltage as a function of temperature as measured by a traditional mercury thermometer. Then, in the second part of the experiment, with their calibration curve in hand, students measure the effect of temperature on the viscosity of two common vegetable oils and compare their findings to literature data.
 |
 |
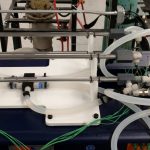
Heat Exchange (CHME 324L)
Description: Two heat exchanger configurations are available. The shell and tube exchanger consists of several tubes in parallel enclosed in a cylindrical shell. Heat is transferred between one fluid flowing through the tubes and another fluid flowing through the cylindrical shell around the tubes. This exchanger demonstrates liquid-to-liquid heat transfer in a 1-7 shell and tube heat exchanger (one shell and seven tubes with two transverse baffles in the shell).
 |
 |
Description: The extended tubular heat exchanger consists of two concentric (coaxial) tubes carrying the hot and cold fluids. The tubes are separated into sections to reduce the overall length and to allow the temperature to be measured at points along both fluid streams.
 |
Analysis of Centrifugal Pumps, Valve Coefficients, and Piping (CHME 323L)
Custom unit manufactured by Unit Ops, Inc.
Description: This apparatus consists of an array of piping, pumps, valves, flow meters, and pressure gauges that permit the pumping of water from a storage tank through several combinations of pumps and valves and back to the storage tank. Students measure pump efficiencies, friction losses in fittings, and valve coefficients.
Study of Fixed and Fluidized Bed Columns (CHME 324L)
 |
Edibon Computer Controlled Fixed and Fluidized Bed Unit
Description: Students study the behavior of vertical glass columns filled to various levels with glass microspheres of different sizes. Pressure drops, effect of microsphere size, and conditions which lead to bed fluidization are studied for columns for which both air and water are used as the fluid medium.
Batch Reaction Engineering (CHME 324L)
 |
A variety of reaction configurations are available.
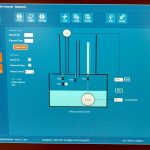
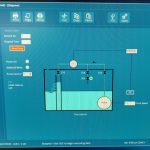
Description: CSTR – The apparatus consists of a small stirred-tank reactor with inlet and outlet flows controlled by peristaltic pumps. The tank is water-jacketed for temperature control with heated water supplied by an adjacent thermostatic-contolled water bath. Reactant streams are pre-heated in this bath as well. The reaction studied is the neutralization of crystal violet by sodium hydroxide, a reaction that can be followed by monitoring the changing color of the solution, which goes from deep purple to clear as the reaction proceeds. The color change is followed by making periodic withdrawals of reactor solution samples and measuring their absorbance with a uv/visible spectrophotometer. For a continuous reactor such as this one, rather than following the reaction to completion, it is followed until steady-state (no further change in absorbance) is achieved. Batch mode operation is also possible.
Plug-Flow Reactor: A small-scale plug-flow reactor (Armfield CEY unit) demonstrates both the flow patter characterization and steady-state conversion in a tubular reactor with axial dispersion. Reaction progress can be followed by either color change or conductivity measurements, depending on the reaction under study.
Laminar Flow Reactor: Similar to the plug-flow reactor described above, this unit (Armfield CEZ) demonstrates flow-pattern characterization and steady-state conversion in a tubular reactor operated under laminar flow conditions.
Gas Absorption Using a Packed Column (CHME 423L)
 |
 |