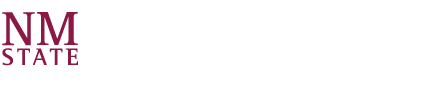This calculation is based on the Dow Lab Safety Module entitled, “Energy Calculations.”
The consequences of not considering the potential energy release of a chemical system includes:
- injury
- loss of primary containment
- equipment failure
- laboratory damage
Consider the example of pressure in a reaction vessel. A researcher is going to study the selective oxidation of 1 mole of o-xylene in a CSTR at 340°C and 285 pig. The reactor has a pressure rating of 2950 pig and a rupture disk with a burst pressure of 2000 psig. Is this relief device necessary?
There are three reactions that occur in this system.
- oX (o-xylene) + 3 O2 → PA (phthalic anhydride), ΔHrxn = -264 kcal/mol
- PA + 7.5 O2 → 8 CO2 + 2 H2O, ΔHrxn = -781 kcal/mol
- oX + 10.5 O2 → 8 CO2 + 5 H2O, ΔHrxn = -1045 kcal/mol
The last reaction represents the worst case scenario (i.e., the largest heat release if oX undergoes total combustion), and is thus used to calculate a reasonable margin of safety.
Using the worst case scenario, estimate the adiabatic temperature rise:
![]()
where
- nA ≡ total moles of reactant in the system
- Cp ≡ heat capacity of the reaction mixture
- XA ≡ conversion (which we assume is 100%)
- Qadiabatic ≡ heat of reaction
Substituting values:
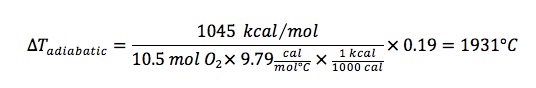
Using a ideal gas equation of state, an estimate of pressure rise can then be made:
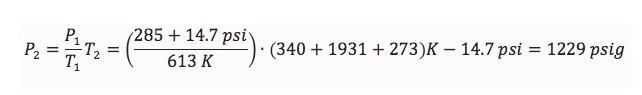
This result suggests a 2000 psig rupture disk setting in a device with a 2950 psig pressure rating represents a relatively safe margin of error.
Watch the video on the Dow safety website.