THE CHALLENGE
Professor Jessica Houston’s research group at New Mexico State University investigates the unique ways in which time-resolved measurements can be used in flow cytometry. An important area of focus for the group is metabolic mapping of cancer cells – assessing their vitality – in the presence of chemotherapeutics, using fluorescent lifetime measurements. Another key application targets fundamental cell signaling and receptor activity, using Förster (fluorescence) resonance energy transfer (FRET) and fluorescence proteins.
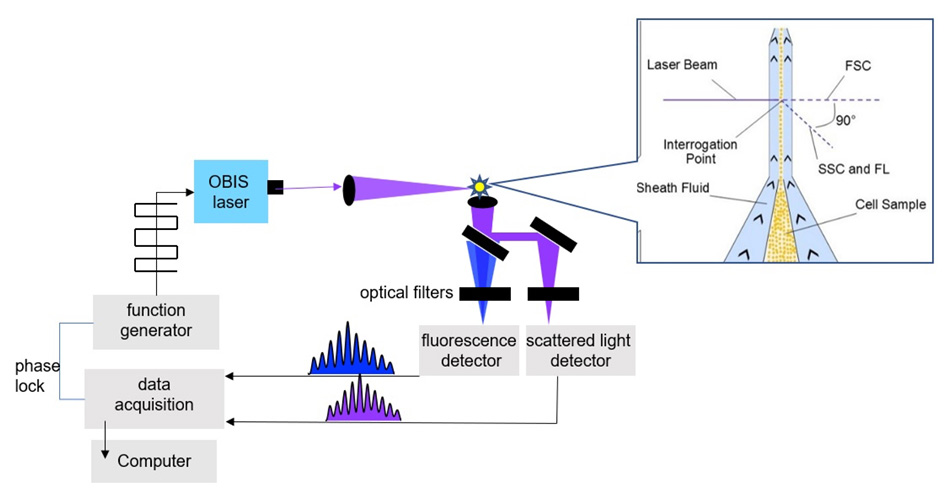
Houston notes that time-resolved flow cytometry is distinctly different to most existing flow cytometry applications which rely on steady-state fluorescence intensity data to perform counting and sorting functions. And the commercially available flow cytometers optimized for steady-state data are not suitable for her research. So as a first step, her group had to build their own flow cytometer.
There are two well-known ways to measure fluorescence lifetimes: in the time domain using a pulsed laser or other light source, or in the frequency domain. Houston explains, “We need a high throughput flow cytometer in order to non-destructively count and/or sort huge populations of cells, e.g., for studying metastatic cancer cells in the blood for example. And time-domain measurements using photon counting would not support the necessary throughput. So we decided to work in the frequency domain, where the established method is to apply a modulation to the excitation laser and correlate the observed phase shift in the modulated fluorescence emission.”
THE SOLUTION
The Houston group wanted the ability to use a lot of different fluorophores in their work in addition to looking at endogenous fluorescence from metabolites like NADH and FAD. So they needed multiple laser wavelengths to efficiently excite all these different targets. They also needed lasers with good mode quality, i.e., low M2, as well as high-stability output, in terms of both beam pointing and power noise.
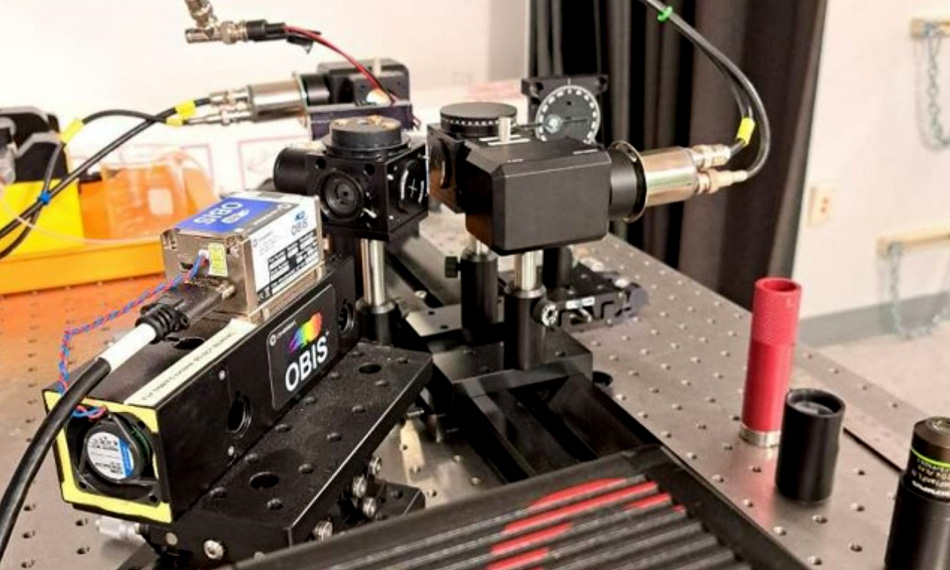
After careful evaluation, they decided to use OBIS lasers from Coherent. Houston notes, “We currently have several OBIS lasers at wavelengths including 375 nm, 405 nm, 488 nm, and 633 nm. These smart lasers are perfect for our work. In addition to their high-quality output, they support the fast direct digital modulation our work needs, plus it is so easy to add and remove these plug & play lasers which all have identical form and fit.” She notes that the availability of 10s and even 100s of milliwatts is another important OBIS feature. This is because so many of her experiments are based on frequency-domain lifetime measurements, where digital modulation essentially drops the laser power at the flow cell by 50%
THE RESULT
Houston states that her custom-built flow cytometer and her growing collection of OBIS lasers have proved to be a successful combination and have already delivered plenty of data for both her metabolic profiling studies as well as the FRET work.
For metabolic profiling, she cites the example of NADH where the measured fluorescence lifetime is dependent on whether this metabolite is primarily in a bound or unbound state in the cell being assessed. This correlates with how the cell is generating ATP, the energetic molecule powering the majority of cellular activity. Metabolic activities in normal cells rely primarily on mitochondrial oxidative phosphorylation (OXPHOS) to generate ATP. But in many types of cancerous cells, glycolysis plays a larger role in ATP production, and OXPHOS capacity is reduced. So the Houston group can use the NADH lifetime as a quantitative metric for how cells are responding to novel treatments for example.
The group are also using the system for fast acquisition of quantitative FRET data. They are analyzing this data to better understand protein-protein interactions, for protein conformational studies, and to probe enzyme actions. Houston notes, “We can look at things like whether a donor fluorophore is in a quenched state of not. We can also capture both donor emissions and receptor emission. Again, this data can indicate the effectiveness of cancer therapeutics. Bottom line, the OBIS lasers and our high-throughput cytometer really are providing a wealth of data.”
References:
- J. Sambrano, F. Rodriguez, J. Martin, and J. P. Houston, "Toward the Development of an On-Chip Acoustic Focusing Fluorescence Lifetime Flow Cytometer," Frontiers in Physics, 2021 9:253 https://www.frontiersin.org/article/10.3389/fphy.2021.647985
- A. Bitton, J. Sambrano, S. Valentino, and J. P. Houston, "A Review of New High-Throughput Methods Designed for Fluorescence Lifetime Sensing From Cells and Tissues," Frontiers in Physics, 2021 9:163 https://www.frontiersin.org/article/10.3389/fphy.2021.648553
- K. Nichani, J. Li, M. Suzuki, and J. P. Houston, “Evaluation of caspase-3 activity during apoptosis with fluorescence lifetime-based cytometry measurements and phasor analyses,” Cytometry A, 2020 97(12):1265-1275; PMC7738394
- F. Alturkistany, K. Nichani, K. D. Houston, and J. P. Houston, “Fluorescence lifetime shifts of NAD(P)H during apoptosis measured by time-resolved flow cytometry,” Cytometry A 2019; 95(1):70-79 PMC6587805
Source: https://www.coherent.com/news/success-stories/new-mexico-state-university